F.Di Bisceglie, E. Lombard, R. Kratzer, N. Gorret, O. Lorain, S.E. Guillouet,
Aerobic autotrophic isopropanol production under low H2 atmosphere in Hollow Fiber Membrane Bioreactor,
Bioresource Technology,
Volume 440,
2026,
133407,
ISSN 0960-8524,
https://doi.org/10.1016/j.biortech.2025.133407.
(https://www.sciencedirect.com/science/article/pii/S0960852425013744)
Abstract: One of the challenges of aerobic gas fermentation for the production of biomolecules is to control the gas environment in order to operate outside the explosive zone, i.e., with low O2 (< 6%) and/or H2 (< 4%) contents. In this study, a bioreactor was to a hollow fiber membrane contactor to supply hydrogen to the microbial suspension. This technology allows the transfer of H2 directly in soluble form into the culture medium without generating bubbles, responsible for the enrichment of the gas mixture with hydrogen by bursting in the gas headspace. Coupling a Hollow Fiber Membrane Contactor to the gas bioreactor in C. necator cultures resulted in either 10 g/L of biomass or 1.3 g/L of isopropanol, while minimizing the H2 content in the gas headspace to a level very close to, or even below, the safety threshold of 4 mol% H2. Isopropanol production performance was compared with that obtained in a gas reactor without HFMC (6.8 g/L of isopropanol). Although the production performance was lower, the coupling still achieved it under low H2 atmosphere, providing proof of concept that it is possible to perform aerobic autotrophic process under low hydrogen atmosphere, thus allowing the exit from the explosive zone regardless of the oxygen content of the reactor. This advancement should help make aerobic gas fermentation much safer and extend these processes on a larger scale.

Cooper, Nina J. Ladevèze, Simon ; Li, Ao ; Shayan, Ramteen ; Fauré, Régis ; Ropartz, David ; Terrapon, Nicolas ; Lombard, Vincent ; Henrissat, Bernard ; Remaud-Siméon ; Magali ; Potocki-Veronese, Gabrielle ; Moulis, Claire ; Cioci, Gianluca ;
Structural and Functional Dissection of GH161 β-Glucan Phosphorylases: Molecular Specificities and Dynamics of Catalysis of Dimeric GH-Q Enzymes
2025/11/21
PY – 2025
DA – 2025/11/21
N1 – doi: 10.1021/acscatal.5c05191
DO – 10.1021/acscatal.5c05191
T2 – ACS Catalysis
JF – ACS Catalysis
JO – ACS Catal.
SP – 19312
EP – 19327
VL – 15
IS – 22
PB – American Chemical Society
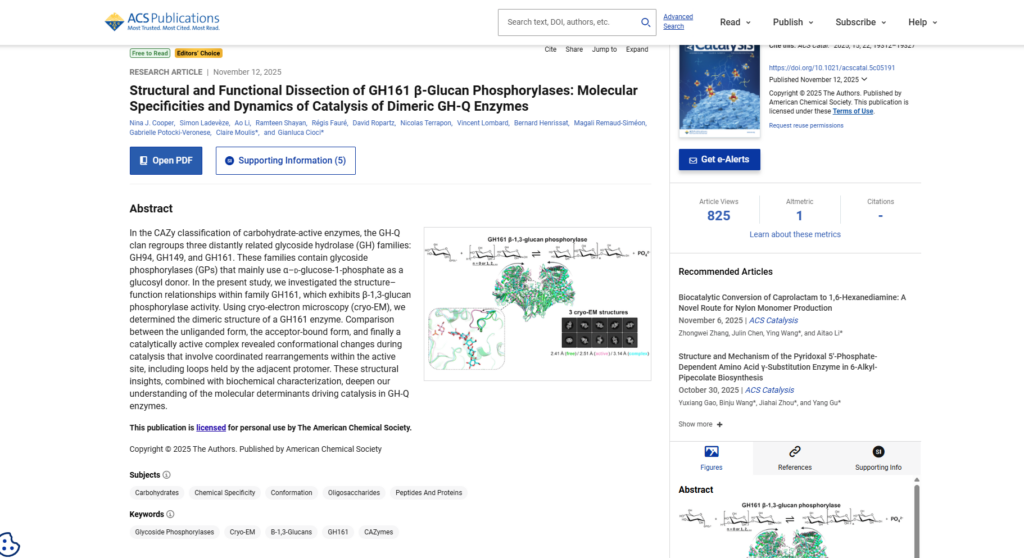
N2 – In the CAZy classification of carbohydrate-active enzymes, the GH-Q clan regroups three distantly related glycoside hydrolase (GH) families: GH94, GH149, and GH161. These families contain glycoside phosphorylases (GPs) that mainly use α–d-glucose-1-phosphate as a glucosyl donor. In the present study, we investigated the structure–function relationships within family GH161, which exhibits β-1,3-glucan phosphorylase activity. Using cryo-electron microscopy (cryo-EM), we determined the dimeric structure of a GH161 enzyme. Comparison between the unliganded form, the acceptor-bound form, and finally a catalytically active complex revealed conformational changes during catalysis that involve coordinated rearrangements within the active site, including loops held by the adjacent protomer. These structural insights, combined with biochemical characterization, deepen our understanding of the molecular determinants driving catalysis in GH-Q enzymes.
AB – In the CAZy classification of carbohydrate-active enzymes, the GH-Q clan regroups three distantly related glycoside hydrolase (GH) families: GH94, GH149, and GH161. These families contain glycoside phosphorylases (GPs) that mainly use α–d-glucose-1-phosphate as a glucosyl donor. In the present study, we investigated the structure–function relationships within family GH161, which exhibits β-1,3-glucan phosphorylase activity. Using cryo-electron microscopy (cryo-EM), we determined the dimeric structure of a GH161 enzyme. Comparison between the unliganded form, the acceptor-bound form, and finally a catalytically active complex revealed conformational changes during catalysis that involve coordinated rearrangements within the active site, including loops held by the adjacent protomer. These structural insights, combined with biochemical characterization, deepen our understanding of the molecular determinants driving catalysis in GH-Q enzymes.
M3 – doi: 10.1021/acscatal.5c05191
UR – https://doi.org/10.1021/acscatal.5c05191